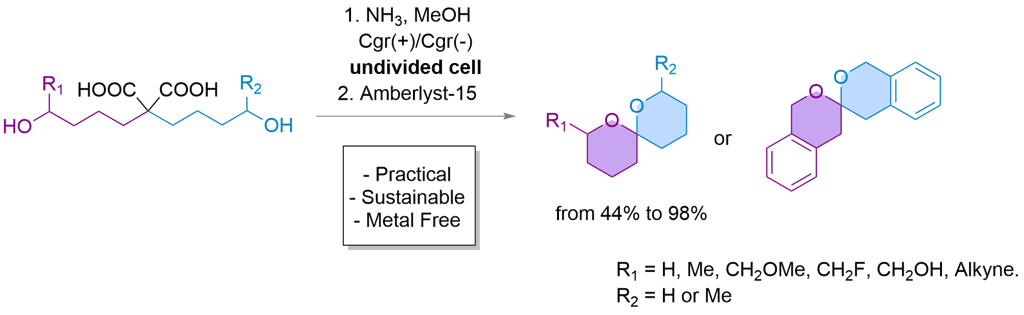Lam Research Group
When Chemistry Has Potential
Welcome
Welcome to our electrifying world of innovative chemistry! Our research is a deep dive into the potential of organic electrosynthesis, where we are pioneering new strategies for synthesising highly reactive intermediates and revolutionising the way we understand and use electrochemistry. We're not just exploring the science, we're reshaping it, using cutting-edge approaches such as flow electrochemistry and pushing the boundaries of medicinal electrosynthesis. Join us on this journey of discovery and innovation as we unlock new potential and redefine the boundaries of chemistry.


Recent Publications
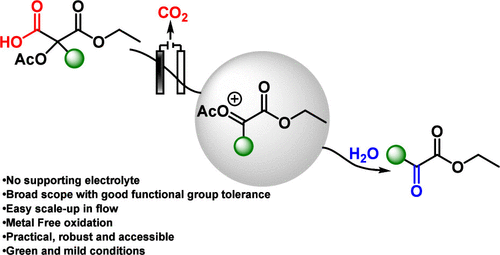
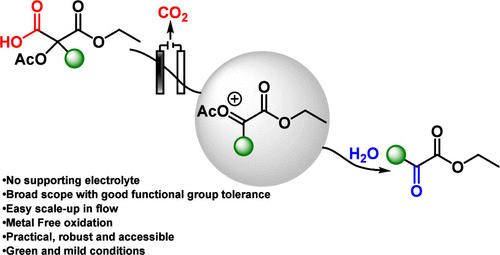
Herein, we report a general and practical electrochemical method for the synthesis of α-keto esters from malonic acid derivatives via α-carbonyl carbocations. Although these cations are typically unstable, they can be efficiently generated under Hofer–Moest-type conditions when stabilized by an α-acetoxy substituent. This metal- and supporting-electrolyte-free approach grants straightforward access to α-keto esters that are otherwise challenging to obtain using conventional methods.
Non-Transition-Metal-Mediated Electrochemical Oxidations of Alcohols to Aldehydes and Ketones
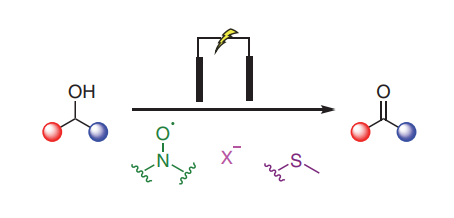
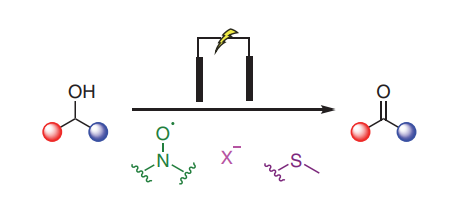
Given the central role of carbonyl compounds in chemical synthesis, considerable effort has been devoted to developing more sustainable and efficient methods for accessing aldehydes and ketones at both laboratory and industrial scales. In recent years electrochemical oxidations of alcohols have seen increased interest in academic settings as a method for removing the toxic and environmentally damaging reagents, such as transition-metal catalysts, found in classical alcohol oxidations. This graphical review aims to deliver a concise summary of the current synthetic electrochemical methods available and place them in the context of the traditional oxidations they aim to replace.
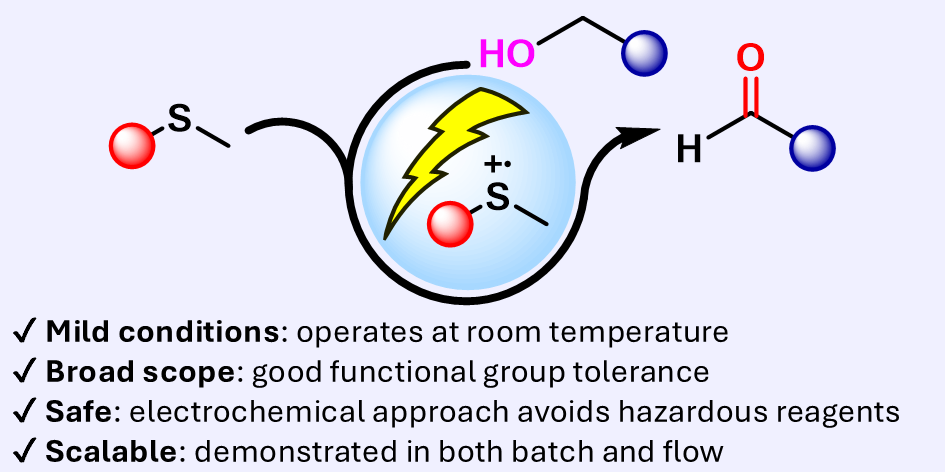
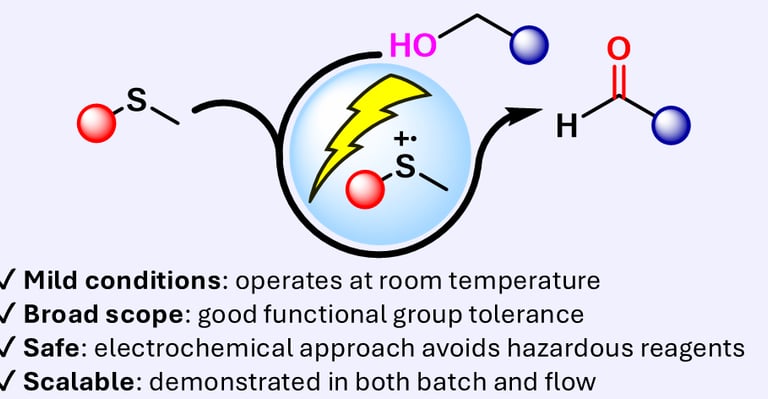
eCarbonyls: an electrochemical thioether mediated oxidation of alcohols to aldehydes and ketones
We report eCarbonyls, a scalable, metal-free electrochemical oxidation of alcohols that mimics key features of the classical Swern reaction while avoiding its reliance on cryogenic conditions and hazardous reagents.
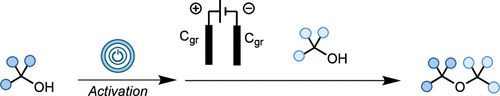
In this work, we disclose a novel electrochemical approach that enables the synthesis of sterically hindered ethers from economically relevant and readily accessible alcohols without the need for sacrificial oxidants. Our protocol exploits mild conditions to generate reactive carbocations, which are subsequently captured by alcohol nucleophiles to yield the desired ethers. This method is cost-effective, practical, and broad in scope, providing a valuable addition to chemists’ synthetic toolkit for ether synthesis.
eEtherification: An Electrochemical Strategy toward the Synthesis of Sterically Hindered Dialkyl Ethers from Activated Alcohols
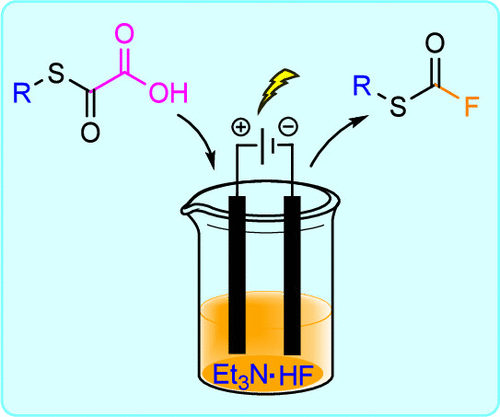
eFluorination for the Rapid Synthesis of Fluorothioformates from Oxalic Acid Monothioesters
An efficient and practical electrochemical method for synthesizing fluorothioformate derivatives, an underexplored functional group, is reported. The strategy is based on the anodic decarboxylation of oxalic acid monothioesters, which rapidly generates highly reactive alkyl (oxomethylidene)sulfonium intermediates.
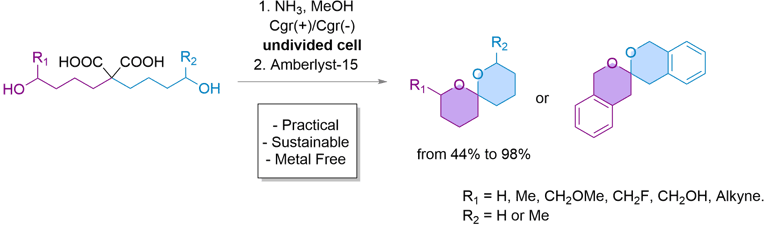
eSpiro: A scalable and sustainable electrosynthetic route to spiroketals via anodic oxidation of malonic acids
Here, we present eSpiro, a novel electrosynthetic method for the efficient and sustainable synthesis of spiroketals via anodic oxidation of malonic acids. This approach offers a metal- and mercury-free alternative to conventional acid-catalysed or transition metal-mediated cyclisations.